Popular heartburn drug may increase risk of pancreatic cancer

Recently, researchers have found that a common drug many individuals take to treat heartburn is linked to a higher risk of pancreatic cancer.
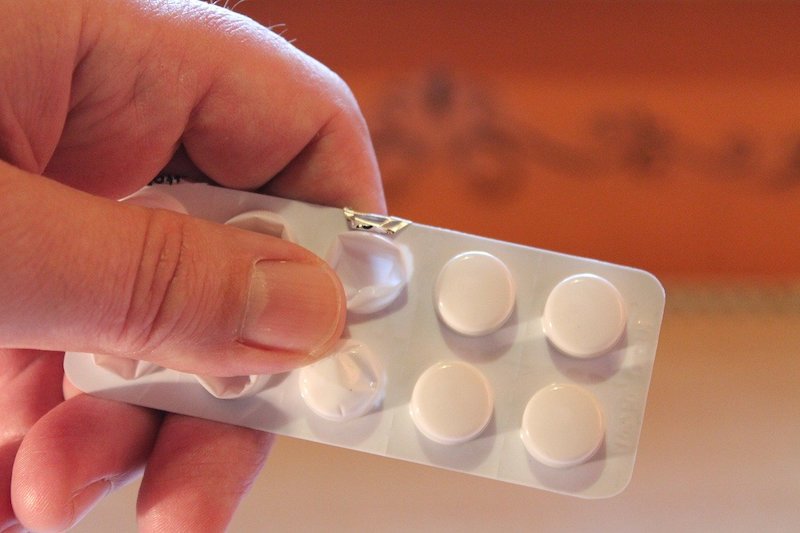
The drug’s name is ranitidine or Zantac.
The Food and Drug Administration requested a manufacturer’s market withdrawal of this drug.
Researchers from Michigan Medicine say that this recall means that all ranitidine products have been removed from the market and will no longer be available for new or existing prescriptions or over-the-counter use.
The results found that the drug may contain a contaminant known as N-nitrosodimethylamine, or NDMA, which is linked to pancreatic cancer and may pose risks to patients.
Other recent studies have found that the longer a product has been sitting around and the more it’s been exposed to high temperatures, the greater the likelihood of the contamination with NDMA.
Not all Zantac drugs are contaminated with NDMA, but currently, there is no way to really determine which ones are and which ones are not.
A gastroenterologist and professor of medicine and nutrition sciences from the University of Michigan discusses the recent ranitidine recall and offers tips for treating the condition now that Zantac is no longer an option.
He suggests that within the same class of H2 receptor antagonists there are a number of others that have not found to have that contaminant.
For example, tagamet or acas Medellin is fine to use. Also, a proton pump inhibitor drug is fine to continue to use.
It is important to remember that H2 blockers tend to work more quickly.
He stresses that all people should stop taking over-the-counter or prescribed Zantac drugs immediately. If they have questions, they should contact their doctors.
Copyright © 2020 Knowridge Science Report. All rights reserved.
This content was originally published here.






